

PEG PRODUCTS

PEGylation Chemistry Info
PEGylation Chemistry Info
-
Example 1 :
PEG-maleimide pegylates thiols of the target compound in which the double bond of the maleimic ring breaks to connect with the thiol. The rate of reaction is pH dependent and best conditions are found around pH 8. But when the pH is higher than 8 and organic co-solvents are present, it has been known cause a side-reaction in which PEG-maleimide may react with amines, although this side-reaction is significantly slower.
-
Example 2 :
PEG-vinylsulfone pegylates thiols of the target compound in the same way as in PEG-maleimide.
-
Example 3 :
PEG-orthopyridyl-disulfide (OPSS) also pegylates thiols resulting in dislfide bond. The conjugation can be decoupled by treatment with reductase such as NaBH4.
-
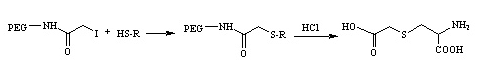
Example 4 :
PEG-iodoacetamide pegylates thiols to form stable thioether bonds in mild basic media. This type of conjugation presents an interesting aspect in that by strong acid analysis the pegylated cysteine residue of the protein can give rise to carboxymethylcysteine which can be evaluated by a standard amino acid analysis (for example, amino acid sequencing), thus offering a method to verify the occurrence of the reaction.
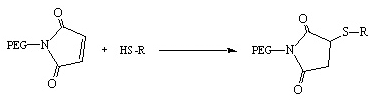
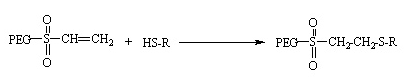
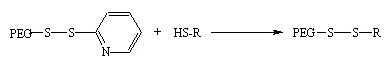

 PEG PRODUCTS
PEG PRODUCTS